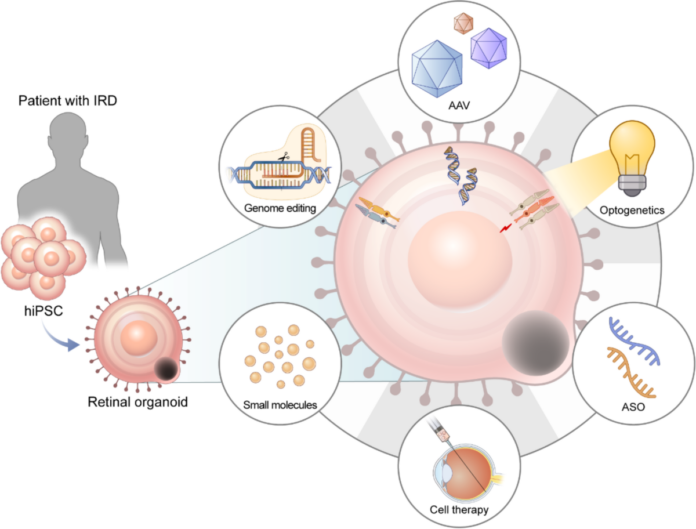
Just over a decade ago, the notion of growing a working eye-in-miniature from reprogrammed skin or blood cells would have met polite scepticism in professional circles. The earliest chapters took shape when pioneering groups in Japan and the UK honed techniques for guiding embryonic and induced pluripotent stem cells into resembling layers of early retinal tissue. You will find that the 2011 demonstration of self-forming optic-cup structures by Yoshiki Sasai’s team set the bar. Over the ensuing years, refinements proliferated: cell culture recipes, improved scaffolds and the addition of subtle growth factors. In the case that you look at recent studies, retinal organoids now display outer nuclear layers rich in rod and cone cells, even demonstrating light responsiveness. Each breakthrough stands on the shoulders of collaborations among bioengineers, clinicians and vision researchers. The technology today reflects an intercontinental relay of innovation, always reaching for tissue that mimics your retina more faithfully.
How Retinal Organoids Are Created
Curiosity might drive you to ask: How do scientists shape cells into complex retinal spheres? The story begins with either embryonic stem cells or induced pluripotent stem cells (iPSCs), the latter created by reprogramming your adult cells back into a flexible, almost-blank slate. If you’re visualising something resembling a chef’s kitchen, you wouldn’t be far off. Cultures are fed precise combinations of nutrients, growth factors and matrices over weeks or even months. These cues nudge stem cells through stages that mirror embryonic eye development, from neuroectoderm layers to eye-field domains, finally forming optic vesicles that bend and coil into what eventually becomes a retina-like cup. Guided, yet very much self-organising, these clusters begin to differentiate into the specific cells you find in a human retina: photoreceptors, bipolar cells, Müller glia and more. In the case that you are examining the timeline, it isn’t swift, the process reflects the gradual choreography you see in fetal eye development. Patience, it seems, is a key ingredient.
Applications of Retinal Organoids in Research
Retinal organoids have quickly become the darling of vision science laboratories, offering tools you might find strangely versatile.
Modelling Eye Diseases with Retinal Organoids
If you will, think of retinal organoids as a stage where gene mutations can play out. Researchers introduce specific genetic changes into stem cells before organoid growth, recreating inherited retinal diseases in vitro. You will find that conditions like retinitis pigmentosa, macular degeneration or congenital stationary night blindness can be studied with these ‘eyes in a dish’. This allows you a unique window into disease onset and progression, free from many of the constraints of animal modelling. The story of your eye’s vulnerabilities can now be told, cell by cell.
Drug Discovery and Toxicology Testing
Before any medicine reaches your local opticians, it must clear a daunting gauntlet of efficacy and safety checks. Retinal organoids give researchers a model for preclinical drug screening. Compounds are applied to organoid cultures to assess their therapeutic effect on photoreceptor health or survival, toxicities and off-target responses become apparent well before any clinical trial. For you, this could mean the possibility of sight-saving therapies arriving more safely and swiftly. In the case that you are following current trends, pharmaceutical companies increasingly view organoids as a reliable testbed.
Potential for Regenerative Medicine and Therapies
You may catch wind of stories in the press about lab-grown tissues restoring vision. Retinal organoids could well sit at the heart of such headlines. Autologous transplantation, where your own cells are turned into retinal organoids and, after careful preparation, re-implanted into an eye affected by disease, sits just over the clinical horizon. Research groups across the UK and Europe have already reported progress in integrating lab-grown photoreceptor cells into animal retinas, with the next logical step being first-in-human trials.
You will find this area especially tantalising, as organoids have the potential to personalise treatment for inherited and acquired retinal dystrophies, which currently have few options. If you know someone with vision loss from macular degeneration or inherited blindness, the possibility of cellular replacement therapies becomes deeply meaningful. In the case that clinical safety can be assured, you might one day be offered a therapy forged from your very own cells.
Current Challenges and Limitations
No new technology reshapes science without a tangle of questions and complexities. You will find that, for all their promise, retinal organoids do not perfectly replicate the adult human retina. Their layered architecture often falls short of the ordered structure in your own eyes, and full vascularisation, the intricate web of tiny blood vessels you need for mature function, remains missing. If you look closely, you might spot immature cells or incomplete synaptic connectivity, resulting in models that are more like embryonic or juvenile tissues than adult retina.
From a practical perspective, the time, cost and expertise required to grow organoids limit their use outside the best-funded labs. Ethical considerations linger too, especially in the case that patient-specific iPSCs are used, demanding careful oversight. You will encounter reports noting variability from batch to batch, which complicates experimental reproducibility. When you read headlines promising new cures, do so with a critical gaze.
Future Directions for Retinal Organoid Research
If curiosity drives you, the horizon seems alive with possibility. The next advances might focus on refining protocols to push organoid development further, adding supporting cells, vascular networks or immune-like features for extra realism. Partnerships between engineers and biologists, so crucial in prior breakthroughs, are now driving new bioreactor technologies, which may one day deliver consistently high-quality retinal organoids on a larger scale.
You might find scientists integrating organoids with microfluidics or transplanting them into bioengineered scaffolds for better growth, even experimenting with gene-editing tools to correct inherited mutations before organoids are created. For those in the UK watching closely, expect further collaboration between NHS hospitals, university labs and biotechnology firms. You could even witness the day when organoids inform routine screening or bespoke therapies for eye disease, a notion as transformative as any in contemporary vision science.
Final Thoughts
You stand at the threshold where biology meets boldness. Retinal organoids, for all their curious charm, symbolise a science that never takes your eyesight for granted. In the years ahead, you will find debate, rightly, over their boundaries and best uses. Yet, the spark they have lit in UK vision research will be hard to extinguish. The next time you hear of a trial using cells from a patient’s own skin to restore sight, know that you are reading the newest chapter in a very old quest: clarity and hope, seeded in a dish, now rippling outward to your world.